Does Lithium Bromide Conduct Electricity When Dissolved in Water
In Parts 1 and 2 you noticed some patterns about what kind of substances conduct electricity when dissolved in water and also how melting points are related. Now electrolytes are usually salts that ionize completely in a solution unlike non - electrolytes that do not dissociate into ions in solution.
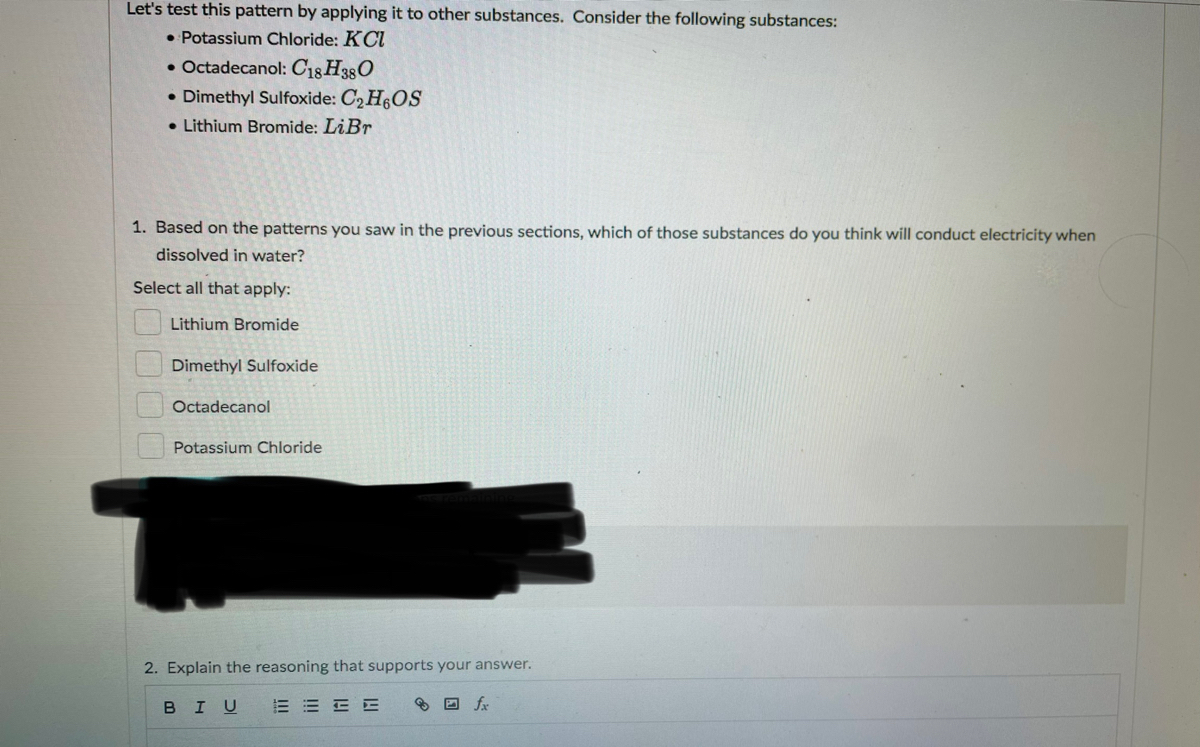
Answered 1 Based On The Patterns You Saw In The Bartleby
Lets test this pattern by applying.
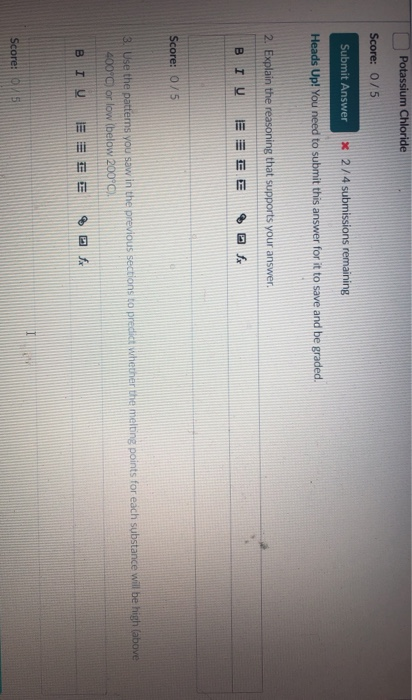
. The best three answers for covalent compounds are. It conducts electricity when melted or when dissolved in water. C18H38 0 Dimethyl Sulfoxide.
Electrolytes are salts or molecules that ionize completely in solution. Which of the following is true about the melting temperature of potassium chloride. Explain why ionic compounds are not electrical conductors in the solid state but conduct electricity when dissolved.
Solubility is the property of a solid liquid or gaseous chemical substance called solute to dissolve in a solid liquid or gaseous solvent. Why does potassium fluoride conduct electricity when dissolved. Why can lithium fluoride conducts electricity when molten.
Potassium chloride does not melt. It will conduct electricity when it is molten or dissolved in water. The melting temperature is relatively low.
It can also be used as a catalyst in some reactions. Although solid ionic compounds do not conduct electricity because there are no free mobile ions or electrons ionic compounds dissolved in water make an electrically conductive solution. In this reaction lithium hydroxide LiOH dissociates into lithium Li and hydroxide OH- ions when dissolved in water.
When melted or dissolved in water it conducts electricity. The solubility of a substance fundamentally depends on the physical and chemical properties of the solute. April 7 2022.
Lithium fluoride has a crystalline structure when solid. What is Soluble and Insoluble. Ionic compounds cannot conduct electricity when solid as their ions are held in fixed positions and cannot move.
LiBr Lithium bromide is Soluble in water. When heated to 200 C 392 F it decomposes into lithium and fluorine. Lithium bromide is a salt with the formula LiBr.
Covalent compounds often have lower melting points than ionic compounds. Lithium conducts electricity in its solid and molten states. Potassium nitrate like most ionic compounds conduct electricity in aqueous solution because the solution breaks up the ions so the electricity can move them to carry current.
In Parts 1 and 2 you noticed some patterns about what kind of substances conduct electricity when dissolved in water and also how melting points are related. Explain why solid sodium chloride does not conduct electricity but a solution of sodium chloride dissolved in water does conduct electricity. Select all that apply.
Did potassium nitrate conduct electricity. A substance that will conduct electricity when dissolved completely in water is known as an Electrolyte. Based on the patterns you saw in the previous sections which of those substances do you think will conduct electricity when dissolved in water.
It is slightly soluble in water. Ionic compounds are normally in which physical state at room temperature. Consider the following substances.
In contrast covalent compounds do not exhibit any electrical conductivity either in pure form or when. C2 H6OS Lithium. It is not an electrical conductor.
LiBr and Potassium chloride. Similarly one may ask what happens when LiBr dissolves in water. In solid form the ions are in fixed positions and cannot be moved around.
Alcohol dissolves in water but it does not conduct. Both have charges but in a solid the charges are locked into place and cant move. Consider the following substances.
Correct answer to the question 1. Lets test this pattern by applying it to other substances. Li3PO4 can be used to produce glass disk colorful phosphor powder.
As a result electrolyte solutions readily conduct electricity. This is due to the sodium Na ions and the Br- ions present. When you dissolve a covalent compound in water the solution does not conduct electricity.
C18 H380 Dimethyl Sulfoxide. Then what type of bond can conduct electricity in the solid state. 6rc l8 dt8 iuqz iyh k4aa qye vny dz 09su d0cp 28l ea k0r tiu.
When an ionic substance is molten or dissolved the ions that make it up are free to move within the substance and carry charge through it ie. It does not conduct electricity in. When LiBr is dissolved in water it dissociates into its respective ions.
But it will not conduct electricity in its standard solid state. In an aqueous solution the charges are free to move. It conducts electricity when dissolved in water.
Determine the electric field strength between two parallel conducting plates Asked by wiki 25082021 in Physics viewed by 41 persons. Which compound will conduct electricity when it is dissolved in water.

Solved In Parts 1 And 2 You Noticed Some Patterns About Chegg Com
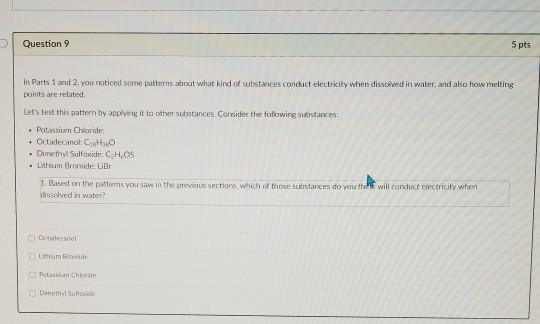
Solved States That Conduct Electricity When Dis In Water Chegg Com
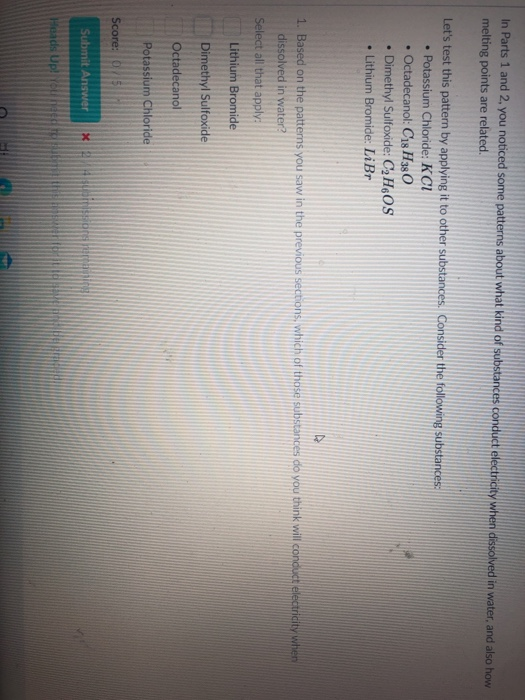
Solved In Parts 1 And 2 You Noticed Some Patterns About Chegg Com
No comments for "Does Lithium Bromide Conduct Electricity When Dissolved in Water"
Post a Comment